What Is the Empirical Formula of C6h6
Empirical formula of a compound A 2 B 4. So ratio of atoms of.

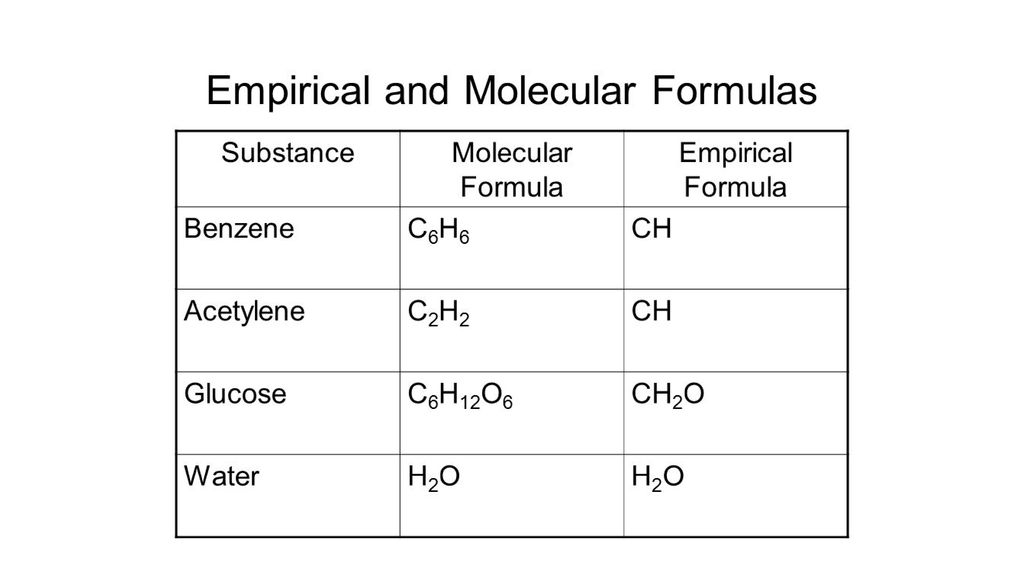
Empirical And Molecular Formulas Ppt Video Online Download
Distinguish between geometric and structural isomers.
. The empirical formula represents the simplest whole number ratio of various atoms in a compound hence for both C 2 H 2 and C 2. Acetic acid C2H4O2 has the same empirical formula as. In chemistry the empirical formula of a chemical compound is the simplest positive integer ratio of atoms present in a compound.
Example- 1 the molecular formula of glucose is C6H12O6. The empirical formula for C6H6 is C3H3. The molecular formula of benzene C₆H₆ tells us that each benzene molecule contains 6 carbon atoms and 6 hydrogen atoms.
What is empirical formula of c6h6. An organic compound on analysis. If its empirical formula weight is half of its vapour density.
Give the Empirical Formula of. For example the molecular formula of glucose is C 6 H 12 O 6 but the empirical formula is CH 2 O. The empirical formula of benzene CH tells us that in each.
Carbon has a valence of 4 oxygen 2. Keeping this in view what is empirical formula of c6h6. A Compound Is 247.
Since the empirical formula is the lowest whole number formula the empirical formula for C6H6 would be CH divide both C and H by 6. CH 66 11. But the empirical formula.
Empirical formula of C6H6. If its empirical formula weight is half of its vapour density. A molecule of glucose contains six atoms of carbon twelve atoms of hydrogen and six atoms of oxygen.
C6H6Cl6 CHClif it seems to be helpful mark me as brainliest. CISCE ICSE Class 9. Up to 24 cash back A compound with an empirical formula of C4H4O and a molar mass of 136 grams per mole.
They are different in any case where the molecular formula may be reduced to a smaller whole-number ratio of elements ex. Molecular formula is the chemical formula which depicts the actual number of atoms of each element present in the compound. If glucose or blood sugar has the molecular formula C 6 H 1 2 O 6 then calculate their empirical formula.
Write the empirical formula for the compound containing carbon to hydrogen in the following ratio 1- 14 2- 26 3-22 4-66. The empirical formula of a compound is C H 2. It represent the simplest whole number ratio of atoms of all the elements present in a.
This is because we can divide each number in C 6 H 12 O 6 by 6 to make a simpler whole. Empirical formula is the simplest chemical. What is the empirical formula of benzene.
Now ratio of Carbon atoms to Hydrogen atoms in Benzene C6H6 is 11. N The molecular formula of benzene is C6H6. Therefore the empirical formula of Benzene is simply CH.

Write The Empirical Formula For The Following C6h6

The Empirical Formula Of Benzene And Acetylene Is Are Youtube

No comments for "What Is the Empirical Formula of C6h6"
Post a Comment